The prevalence of diabetes has been on the rise in recent years. Pancreatic beta cells, which produce insulin, have been central to the condition. Scientists have been employing them to find new therapeutic targets and formulate more effective treatment modalities. Research has focused on delineating signaling pathways pertaining to the development of beta islets, their functions, and physiological processes.
Pancreatic Islet Beta Cells
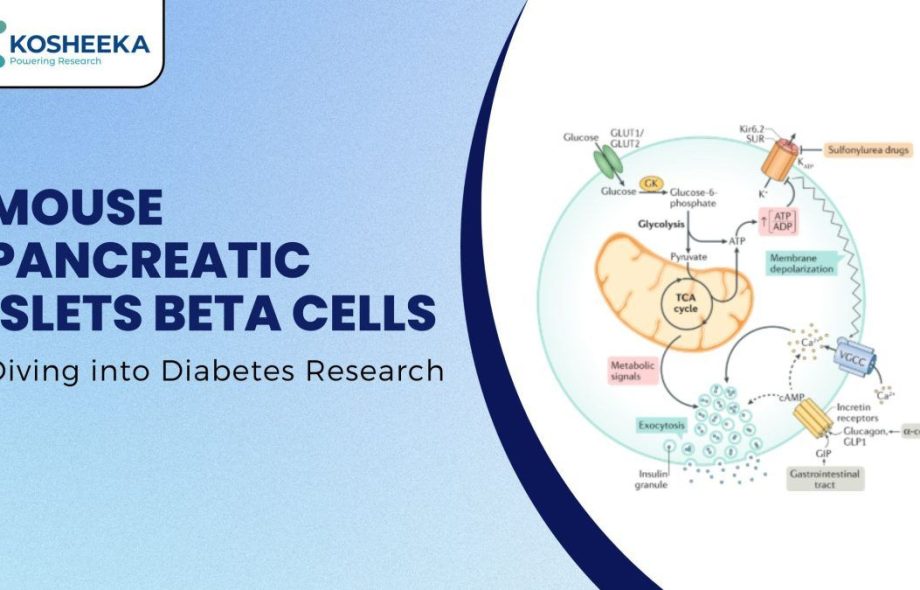
The pancreas is an endocrine as well as an exocrine gland. Its exocrine functions are associated with the gastrointestinal tract. It has cellular aggregates or islands called Islets of Langerhans, after the person who discovered them for endocrine functions. These islets comprise different kinds of cells- alpha, beta, gamma, delta, and epsilon. Pancreatic Beta Cells produce insulin and secrete it in a glucose-responsive manner; that is, they release insulin in response to blood glucose levels.
Additionally, stimuli such as nutrients (fatty acids), hormones (gastrin), and neurotransmitters (acetylcholine) induce the secretion of insulin. The secretion process of insulin follows the transport of glucose in the beta islets and their metabolism, which generates ATP. ATP closes ATP-based potassium ion channels, leading to membrane depolarization, resulting in calcium influx that triggers exocytosis of insulin granules.
Beta Cell Physiology
Insulin controls blood glucose levels by increasing its absorption by body cells. It increases the expression of glucose transporters on cell membranes to enhance their transfer into cells. Therefore, an inadequate amount of insulin leads to diabetes. Type I diabetes describes the destruction of beta islets by the immune system and type II diabetes is marked by insulin resistance by body cells.
Beta cells can proliferate or undergo apoptosis, depending on the physiological demands. Its turnover increases in conditions like obesity, pregnancy, and insulin resistance. Their survival and proliferation depend on pathways like mTOR and PI3K/PKB. MAFA, NK6 homoeobox 1, and PDX1 have been identified as key transcription factors in the beta cell development process. Inflammation and oxidative stress trigger their apoptosis.
Mouse Pancreatic Islets Beta Cells
Mouse models have been pivotal for in vivo research. Their similarity to humans, short lifespan, compatibility with gene editing, cost-effective breeding, and large litter size have facilitated their use. Their inbred strains confer them homogeneity. The commonly used mouse models for diabetic research are:
NOD (Non-Obese Diabetic) Mice: It is frequently used for type I Diabetes Research due to their susceptibility to diabetes.
Db/db mice: These mice have a genetic mutation in the gene for leptin receptors, resulting in dysfunctional leptin receptors. The leptin receptor regulates energy homeostasis, thus causing obesity and subsequent hyperglycemia in db/db mice. Therefore, they are utilized for type II diabetes research.
Ob/ob mice: Due to their genetic deletion, these models lack leptin receptors and are, therefore, suitable for diabetes research.
Such mouse models have provided deep insights into disease pathology. Therefore, mouse pancreatic islet beta cells are apt for in vitro research.
Three-Dimensional Culture
The three-dimensional (3D) culture of cells has been gaining popularity in the past decade. This model recapitulates the in vivo system more accurately, accounting for cell-cell interaction and spatial variations in the tissue microenvironment. A few companies have already invested in creating and supplying such a 3D culture of beta islet cells. The creation of different scaffolds and understanding of their interaction with cells have been promoting the generation of 3D culture systems. These can serve as suitable platforms for studying cellular processes and screening novel drugs.
Dedifferentiation
The loss of beta islets occurs due to apoptosis. Interestingly, recent studies have reported that aside from apoptosis, beta islets undergo dedifferentiation into progenitors. Due to environmental stress, they reduce the expression of beta cell-specific genes and upregulate precursor genes. In addition to dedifferentiation, they also exhibit transdifferentiation into alpha cells. Scientists are investigating these processes to design therapies that can reverse these processes and restore insulin secretion.
Bioartificial Pancreas
Researchers have devised several strategies to treat diabetes, such as bioartificial pancreas. The concept of bioartificial pancreas focuses on encapsulating pancreatic beta cells within semi-permeable membranes and transplanting them inside. Encapsulation reduces the immune interaction and provides insulin in a natural manner. In vivo and in vitro studies have supported their applications. Macroscale devices such as betaAir Bio, artificial pancreas, and Encaptra have employed the encapsulation technique but have low nutrient transport capacity. Microscale devices have overcome this obstacle. Clinical trials of these devices might soon prove their efficacy and safety.
Final Thoughts
Pancreatic beta cells have been vital for diabetes research. They have essentially shaped our understanding of diabetes. Ongoing research has been revealing new signaling pathways and cellular events that contribute to the disease pathogenesis. 3D models will significantly improve our knowledge base in the future. Several treatment strategies revolving around these cells are showing promising potential. As the research on these cells continues, a more effective therapy will surface. Kosheeka assists you in your study by supplying top-grade cells with thorough characterization and robust testing.
 :
https://in.pinterest.com/kosheeka/
:
https://in.pinterest.com/kosheeka/